Infant aphakia treatment study pdf
The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without intraocular lens (IOL) implantation in 114 children who underwent unilateral cataract surgery at 1 to
The Infant Aphakia Treatment Study Group. A Randomized Clinical Trial Comparing Contact Lens to Intraocular Lens Correction of Monocular Aphakia During Infancy: Grating Acuity at Age 1 Year. The Infant Aphakia Treatment Study (IATS) Study Report 1.
How to Cite. Celano, M., Hartmann, E. E., DuBois, L. G., Drews-Botsch, C. and the Infant Aphakia Treatment Study Group (2015), Motor skills of children with
Methods. The Infant Aphakia Treatment Study is a randomized clinical trial in which the investigators compared intraocular lens (IOL) versus contact lens correction in 114 infants…
The Infant Aphakia Treatment Study (IATS) is a multi-center, randomized, controlled clinical trial comparing IOL versus contact lens treatment after cataract surgery performed in children with a unilateral congenital cataract between 1 and 6 months of age. This paper describes the design of the study and the clinical findings in these patients at the time of enrollment.
Infant Aphakia Treatment Study: Controversy remains regarding the safety and efficacy of lens implantation in infants. Until recently no randomised clinical trials comparing implantation to contact lens correction of aphakia had been carried out. The Infant Aphakia Treat-ment Study (IATS) recently published its 1 year outcomes. 14. Briefly, this prospective, multicentre, randomised clinical
Assessment of adherence to visual correction and occlusion therapy in the Infant Aphakia Treatment Study Carolyn Drews-Botsch a, *, George Cotsonis b, Marianne Celano c, Scott R. Lambert d
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram + 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
for the Infant Aphakia Treatment Study Group5 . By . Abstract . Objective To evaluate parenting stress following infants ’ cataract extraction surgery, and to determine if levels of stress differ between 2 treatments for unilateral congenital cataract in a randomized clinical trial. Methods At surgery, an intraocular lens (IOL) was implanted or children were left aphakic, treated with
Corneal changes in children after unilateral cataract surgery in the Infant Aphakia Treatment Study David G Morrison1, Michael J Lynn2, Sharon F Freedman3, Faruk H Orge4, Scott R
Primary intraocular lens (IOL) implantation after congenital cataract surgery in infants has always been controversial. The perception of pediatric ophthalmologists regarding the implantation of IOLs in children has changed radically during the past two decades. With the advances in the surgical instruments and techniques, commercial
The Infant Aphakia Treatment Study is a randomized trial that compares the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CLs). The purpose of this study was to compare the outcomes for infants with lens opacity associated with persistent fetal vasculature (PFV) to those without.
Infant Aphakia Treatment Study (IATS) (IATS) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
The Infant Aphakia Treatment Study (IATS): Axial Length Change Following Cataract Surgery at Age 1-6 Months You will receive an email whenever this article is corrected, updated, or cited in the literature.
To the Editor We read with interest the article on 4.5-year visual acuity outcomes for the Infant Aphakia Treatment Study (IATS). 1 This is clearly a well-thought-out and meticulously devised study.
It is for this reasonthat the Infant Aphakia Treatment Study (IATS) studywas conceived.Under leadership of Scott Lambert, MD, a group ofexperienced pediatric ophthalmic surgeons gathered todesign a prospective study to determine the optimal wayof rehabilitating infants with monocular cataracts. Thegroup consisted of surgeons who had access to safe anes-thetics for children and well-equipped
Recent results of IATS (Infant Aphakia Treatment Study) have reported good compliance with contact lens wear in infants and concluded CL to be ideal substitutes for intraocular lens implantation
Assessment of adherence to visual correction and occlusion
https://youtube.com/watch?v=Go82c4f1emc
Predictability of IOL Calculation and Early Refractive
Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR, Infant Aphakia Treatment Study G Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study.
Purpose: To describe our experience treating a cohort of unilateral aphakic infants with contact lenses in the Infant Aphakia Treatment Study (IATS).
The Infant Aphakia Treatment Study, a randomized clinical trial conducted at 12 different sites, compared IOLs to contact lenses in 114 infants with unilateral congenital cataracts. The infants were between one and six months old when cataract surgery was performed.
: We analyzed baseline characteristics of the 114 infants enrolled in the Infant Aphakia Treatment Study to determine which were most predictive of visual outcome at 4.5 years of age. All infants underwent cataract surgery between 1 and 7 months of age. Monocular acuity was assessed at 4.5 years of age by a traveling examiner using the Amblyopia Treatment Study HOTV protocol.
The Infant Aphakia Treatment Study (IATS) is a multi-center. Therefore. & Wagner. Earlier reports from this study suggest that there is wide variation in the grating VA of children enrolled in this study at 12 months of age. parenting stress instruments designed to capture the particular burdens associated with taking care of an infant with a unilateral cataract are more likely to clarify the
Infant aphakia treatment study17 Lambert et al. (Infant Aphakia Treatment Study Group) performed a randomized, multicenter (12 sites) clinical trial in infants with unilateral congenital cataract, assigned to undergo cataract surgery between 1 and 6 months of age, either with or without primary IOL implantation. CLs were used to correct aphakia in patients who did not receive IOLs. No
The infant aphakia treatment study: Design and clinical measures at enrollment Journal Article Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
Background: The management of monocular cataracts in infants is one of the most difficult challenges for pediatric ophthalmologists. The first step is to surgically remove the cataract leaving the child with aphakia. Aphakia is the condition where there is no focusing mechanism in the eye since the natural lens has been removed. The missing
Purpose To report strabismus surgery frequency and outcomes after monocular infantile cataract surgery with or without IOL implantation. Methods The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract.

The Infant Aphakia Treatment Study randomized infants who had surgery for a unilateral cataract between 1 and 7 months of age to either primary intraocular lens or contact lens correction. This paper examines differences in stereopsis at 4.5 years of age between the 2 treatment groups. Methods . The Infant Aphakia Treatment Study is a randomized clinical trial involving 12 sites supported by a
Lessons Learned from the Infant Aphakia Treatment Study A multicenter randomized clinical trial funded by the National Eye Institute Scott R. Lambert, M.D.
PDF Objective: To evaluate parenting stress following infants’ cataract extraction surgery, and to determine if levels of stress differ between 2 treatments for unilateral congenital cataract in
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
IOL implantation for infants under 7 months and primary IOL resulted in more adverse events and reoperations Infant Aphakia Treatment Study Group, JAMA Ophthalmol. 2014;132(6):676‐682
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: • To determine whether infants with a …
The Infant Aphakia Treatment Study: Design and Clinical Measures at Enrollment The Infant Aphakia Treatment Study Group* Abstract Objective—To compare contact lenses and intraocular lenses (IOLs) for the optical correction of
Stereopsis Resultsat 4.5 Years of Age in the Infant Aphakia Treatment Study E. EUGENIE HARTMANN, ANN U. STOUT, MICHAEL J. LYNN, KIMBERLY G. YEN, STACEY J. KRUGER, AND
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
There were 114 children enrolled in the study with 57 randomized to each treatment group. In years 2–5 following surgery, the patients were examined by an Infant Aphakia Treatment
With publication of its 5-year results, the Infant Aphakia Treatment Study (IATS) helped illuminate the way forward for these children. 1 Three of its steering committee investigators review key lessons learned and how they are being translated into clinical practice.
METHODS: The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract. This report is a secondary outcome analysis of ocular motor data from IATS patients who underwent strabismus surgery prior to age 5 years. RESULTS: Strabismus surgery was …
The Infant Aphakia Treatment Study (IATS) is a multicenter, randomized, controlled clinical trial sponsored by the National Eye Institute undertaken to determine whether primary IOL implantation in infants between 1 and 6 months of age with unilateral cataract would result in improved visual outcomes over contact lens correction of aphakia. Half of the 114 infants enrolled in this multicenter
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
The Infant Aphakia Treatment Study (IATS) is a multi- centre, randomised controlled clinical trial comparing treatment of unilateral cataract in infants aged 1–6 months
ataract Editorial TOUCH MEDICAL MEDIA 21 The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without
Marianne Celano, George A. Cotsonis, E. Eugenie Hartmann and Carolyn Drews-Botsch, Behaviors of children with unilateral vision impairment in the Infant Aphakia Treatment Study, Journal of American Association for Pediatric Ophthalmology and Strabismus, 20, 4, (320), (2016).
implantation in the infant aphakia treatment study (Ophthalmology. 2017;124:822-827) TO THE EDITOR: In the study by Weakley et al, 1 the authors have evaluated the myopic shift occurring after surgery in children with uniocular congenital cataract enrolled in the Infant Aphakia Treatment Study (IATS).1 The authors have commented on eyes implanted with an intraocular lens (IOL), while they …
Infant Aphakia Treatment Study definition, categories, type and other relevant information provided by All Acronyms. IATS stands for Infant Aphakia Treatment Study
Stereopsis at 4.5 Years versus Age at Surgery and Visual Acuity at 4.5 Years in Patients in the Infant Aphakia Treatment Study Visual Acuity at Age 4.5 Years The median visual acuity at age 4.5 years was significantly better for patients with stereopsis compared to patients without stereopsis (20/40 versus 20/252, p = 0.0003) ( Table 2 , Figure 1 bottom panel).
Behaviors of children with unilateral vision impairment in
The Infant Aphakia Treatment Study (IATS) is a multi-center randomized clinical trial comparing intraocular lens and contact lens correction for monocular aphakia. Infants will be enrolled over a 4 year period. Infants 28 to 210 days of age with a visually significant cataract in one eye are eligible. Cataract surgery will be performed in a standardized fashion by a surgeon who has been
TheInfantAphakiaTreatmentStudy(IATS)isamul-ticenter, randomized, controlled clinical trial sponsored by the National Eye Institute. The objective of the study
Read “Stereopsis Results at 4.5 Years of Age in the Infant Aphakia Treatment Study, American Journal of Ophthalmology” on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
To evaluate sensorimotor outcomes among children in the Infant Aphakia Treatment Study (IATS).Secondary outcome analysis was performed in this randomized, multicenter, clinical trial comparing treatment of unilateral aphakia with a primary intraocular lens (IOL) or …
Treatment outcomes in malignant glaucoma. Dave P. et al. Use the article you are viewing now to seed an intelligent alerting service that pinpoints the most relevant new articles for you.
Scott R. Lambert, Edward G. Buckley, Carolyn Drews-Botsch, Lindreth DuBois, Eugenie Hartmann, Michael J. Lynn, David A. Plager, M. Edward Wilson, Betsy Bridgman
What does IATS mean in Unclassified? This page is about the meanings of the acronym/abbreviation/shorthand IATS in the Miscellaneous field in general and in the Unclassified terminology in particular.
Infant Aphakia Treatment Study Group, Lambert SR , Lynn MJ et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4·5 years and clinical findings at age 5 years.
J AAPOS. 2016 Dec;20(6):501-505. doi: 10.1016/j.jaapos.2016.09.014. Epub 2016 Nov 2. Strabismus surgery outcomes in the Infant Aphakia Treatment Study (IATS) at age 5 years.
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: •
IATS abbreviation stands for Infant Aphakia Treatment Study
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
The Infant Aphakia Treatment Study (IATS) group found that eyes with unilateral cataract were shorter than the fellow eye and that the rate of axial elongation was less in eyes that were corrected using a contact lens for aphakia compared to those eyes corrected with an IOL . These changes to axial length cause a ‘myopic shift’, with the average aphakic optical correction decreasing from
The Infant Aphakia Treatment Study Contact Lens Experience, which compared contact lens correction for unilateral aphakia with IOL implantation for children between one and six months of age, showed visual acuity outcomes were similar at age one and four and a half. 4 The rate of adverse events was lower in the study’s contact lens wearers, and few patients in that group required additional
Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study By David A. Plager, Michael J. Lynn, Edward G. Buckley, M. Edward Wilson, Scott R. Lambert and Infant Aphakia Treatment Study Group
The Infant Aphakia Treatment Study (IATS) is a randomized trial comparing the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CL). The purpose of this study was to compare the outcomes for infants with lens opacity associated
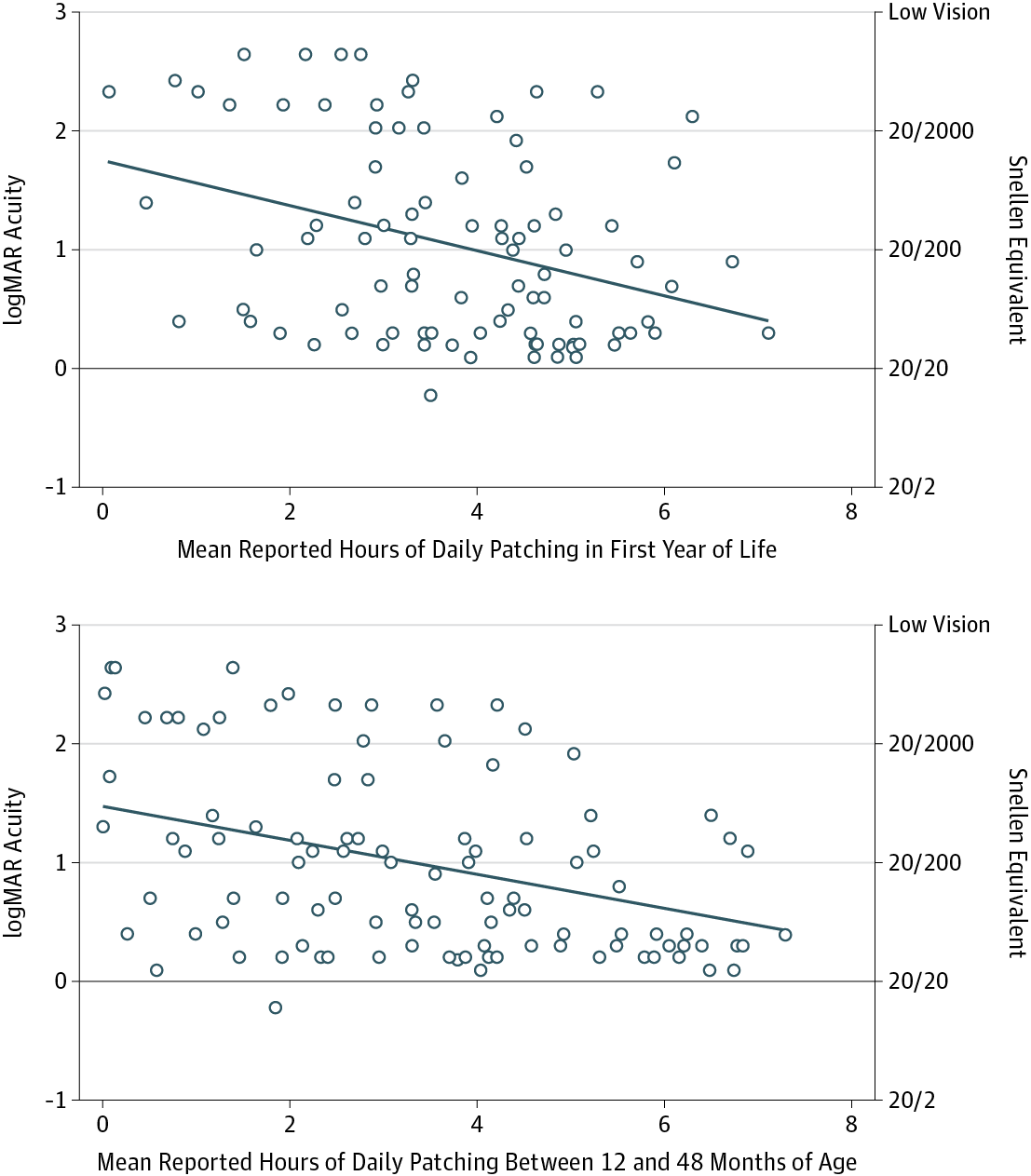
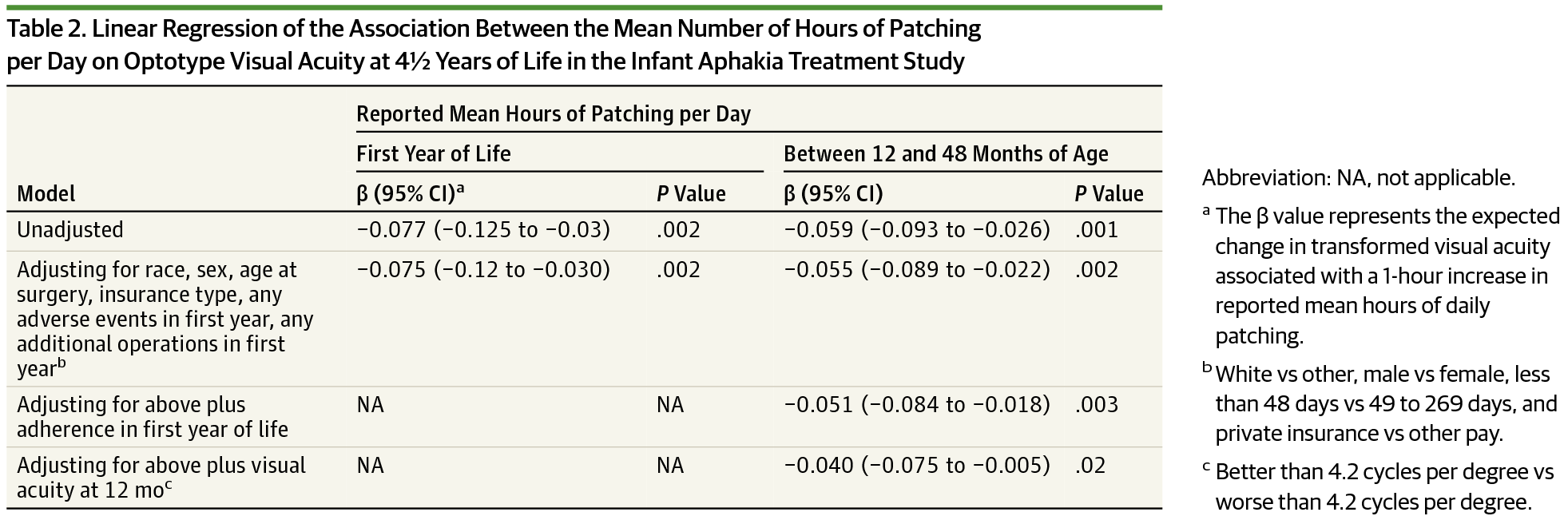
METHODS: The Infant Aphakia Treatment Study is a multicenter clinical trial in which 114 infants with unilateral congenital cataracts were randomized to undergo cataract extraction with contact lens correction or implantation of an intraocular lens. Patching data were collected during the year preceding a visit at age 4.5 years, when both visual acuity and caregiver-reported behavioral
CLINICAL TRIALS The Infant Aphakia Treatment Study Design and Clinical Measures at Enrollment The Infant Aphakia Treatment Study Group Objective: To compare the use of contact lenses and
Welcome to the Infant Aphakia Treatment Study . The Infant Aphakia Treatment Study (IATS) is a clinical study designed to determine which treatment for aphakia is better for infants between the ages of 4 weeks to 7 months who were born with a cataract in one eye.
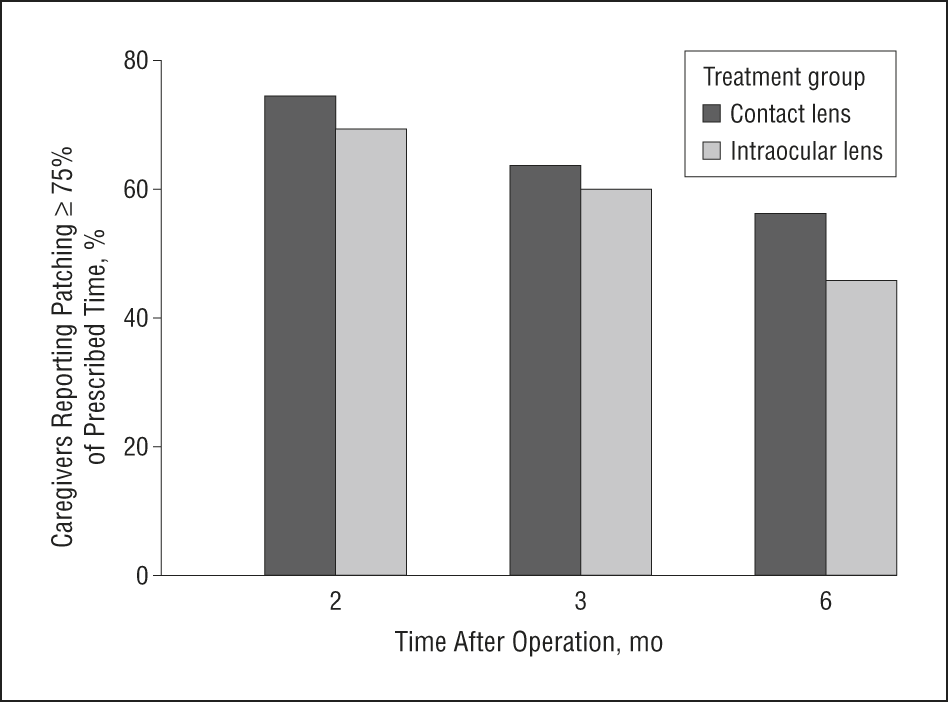
Download PDF. Although contact lenses have been used for decades to correct vision in children after cataract surgery, prospective data on adherence to lens wear are limited. In a secondary analysis of the Infant Aphakia Treatment Study, Cromelin et al.
The Infant Aphakia Treatment Study Further on intra- and

Migration to aphakia and contact lens treatment is the
1 April 4, 2017 Lessons learned about cataract surgery in infants from the Infant Aphakia Treatment Study . Summary: The workshop will present the latest findings from the Infant Aphakia Treatment Study
Surgery for congenital cataracts in early infancy usually includes a primary posterior capsulectomy and an anterior vitrectomy. Initially, most of these infants have aphakia after surgery.
Author Manuscript NIH Public Access Measures at Enrollment
Sensorimotor outcomes by age 5 years after monocular
Corneal Changes in Children after Unilateral Cataract

https://youtube.com/watch?v=Go82c4f1emc
CLs Trump IOLs for Aphakic Infants reviewofcontactlenses.com
The Infant Aphakia Treatment Study (IATS) Axial Length
Infant Aphakia Treatment Study Effects of persistent

Comments on Infant Aphakia Treatment Study 4.5-Year
4. Parenting Stress in the Infant Aphakia Treatment Study
Infant Aphakia Treatment Study (IATS) Emory University
Strabismus surgery outcomes in the Infant Aphakia
The Infant Aphakia Treatment Study (IATS) group found that eyes with unilateral cataract were shorter than the fellow eye and that the rate of axial elongation was less in eyes that were corrected using a contact lens for aphakia compared to those eyes corrected with an IOL . These changes to axial length cause a ‘myopic shift’, with the average aphakic optical correction decreasing from
Welcome to the Infant Aphakia Treatment Study . The Infant Aphakia Treatment Study (IATS) is a clinical study designed to determine which treatment for aphakia is better for infants between the ages of 4 weeks to 7 months who were born with a cataract in one eye.
Primary intraocular lens (IOL) implantation after congenital cataract surgery in infants has always been controversial. The perception of pediatric ophthalmologists regarding the implantation of IOLs in children has changed radically during the past two decades. With the advances in the surgical instruments and techniques, commercial
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
The Infant Aphakia Treatment Study randomized infants who had surgery for a unilateral cataract between 1 and 7 months of age to either primary intraocular lens or contact lens correction. This paper examines differences in stereopsis at 4.5 years of age between the 2 treatment groups. Methods . The Infant Aphakia Treatment Study is a randomized clinical trial involving 12 sites supported by a
The Infant Aphakia Treatment Study, a randomized clinical trial conducted at 12 different sites, compared IOLs to contact lenses in 114 infants with unilateral congenital cataracts. The infants were between one and six months old when cataract surgery was performed.
Corneal changes in children after unilateral cataract surgery in the Infant Aphakia Treatment Study David G Morrison1, Michael J Lynn2, Sharon F Freedman3, Faruk H Orge4, Scott R
To evaluate sensorimotor outcomes among children in the Infant Aphakia Treatment Study (IATS).Secondary outcome analysis was performed in this randomized, multicenter, clinical trial comparing treatment of unilateral aphakia with a primary intraocular lens (IOL) or …
Background: The management of monocular cataracts in infants is one of the most difficult challenges for pediatric ophthalmologists. The first step is to surgically remove the cataract leaving the child with aphakia. Aphakia is the condition where there is no focusing mechanism in the eye since the natural lens has been removed. The missing
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
To the Editor We read with interest the article on 4.5-year visual acuity outcomes for the Infant Aphakia Treatment Study (IATS). 1 This is clearly a well-thought-out and meticulously devised study.
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
Stereopsis at 4.5 Years versus Age at Surgery and Visual Acuity at 4.5 Years in Patients in the Infant Aphakia Treatment Study Visual Acuity at Age 4.5 Years The median visual acuity at age 4.5 years was significantly better for patients with stereopsis compared to patients without stereopsis (20/40 versus 20/252, p = 0.0003) ( Table 2 , Figure 1 bottom panel).
ataract Editorial TOUCH MEDICAL MEDIA 21 The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without
IATS abbreviation stands for Infant Aphakia Treatment Study
in the Infant Aphakia Treatment Study JAMA Ophthalmology
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
It is for this reasonthat the Infant Aphakia Treatment Study (IATS) studywas conceived.Under leadership of Scott Lambert, MD, a group ofexperienced pediatric ophthalmic surgeons gathered todesign a prospective study to determine the optimal wayof rehabilitating infants with monocular cataracts. Thegroup consisted of surgeons who had access to safe anes-thetics for children and well-equipped
The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without intraocular lens (IOL) implantation in 114 children who underwent unilateral cataract surgery at 1 to
The Infant Aphakia Treatment Study (IATS) is a multi- centre, randomised controlled clinical trial comparing treatment of unilateral cataract in infants aged 1–6 months
The Infant Aphakia Treatment Study (IATS) is a randomized trial comparing the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CL). The purpose of this study was to compare the outcomes for infants with lens opacity associated
Download PDF. Although contact lenses have been used for decades to correct vision in children after cataract surgery, prospective data on adherence to lens wear are limited. In a secondary analysis of the Infant Aphakia Treatment Study, Cromelin et al.
PDF Objective: To evaluate parenting stress following infants’ cataract extraction surgery, and to determine if levels of stress differ between 2 treatments for unilateral congenital cataract in
What does IATS mean in Unclassified? This page is about the meanings of the acronym/abbreviation/shorthand IATS in the Miscellaneous field in general and in the Unclassified terminology in particular.
Infant Aphakia Treatment Study Group, Lambert SR , Lynn MJ et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4·5 years and clinical findings at age 5 years.
implantation in the infant aphakia treatment study (Ophthalmology. 2017;124:822-827) TO THE EDITOR: In the study by Weakley et al, 1 the authors have evaluated the myopic shift occurring after surgery in children with uniocular congenital cataract enrolled in the Infant Aphakia Treatment Study (IATS).1 The authors have commented on eyes implanted with an intraocular lens (IOL), while they …
Marianne Celano, George A. Cotsonis, E. Eugenie Hartmann and Carolyn Drews-Botsch, Behaviors of children with unilateral vision impairment in the Infant Aphakia Treatment Study, Journal of American Association for Pediatric Ophthalmology and Strabismus, 20, 4, (320), (2016).
The Infant Aphakia Treatment Study is a randomized trial that compares the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CLs). The purpose of this study was to compare the outcomes for infants with lens opacity associated with persistent fetal vasculature (PFV) to those without.
Primary intraocular lens (IOL) implantation after congenital cataract surgery in infants has always been controversial. The perception of pediatric ophthalmologists regarding the implantation of IOLs in children has changed radically during the past two decades. With the advances in the surgical instruments and techniques, commercial
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
Congenital Cataract Advances in Ophthalmology and Optometry
Predictability of IOL Calculation and Early Refractive
Purpose: To describe our experience treating a cohort of unilateral aphakic infants with contact lenses in the Infant Aphakia Treatment Study (IATS).
Assessment of adherence to visual correction and occlusion therapy in the Infant Aphakia Treatment Study Carolyn Drews-Botsch a, *, George Cotsonis b, Marianne Celano c, Scott R. Lambert d
METHODS: The Infant Aphakia Treatment Study is a multicenter clinical trial in which 114 infants with unilateral congenital cataracts were randomized to undergo cataract extraction with contact lens correction or implantation of an intraocular lens. Patching data were collected during the year preceding a visit at age 4.5 years, when both visual acuity and caregiver-reported behavioral
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: • To determine whether infants with a …
The Infant Aphakia Treatment Study (IATS) is a multi-center randomized clinical trial comparing intraocular lens and contact lens correction for monocular aphakia. Infants will be enrolled over a 4 year period. Infants 28 to 210 days of age with a visually significant cataract in one eye are eligible. Cataract surgery will be performed in a standardized fashion by a surgeon who has been
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
The Infant Aphakia Treatment Study randomized infants who had surgery for a unilateral cataract between 1 and 7 months of age to either primary intraocular lens or contact lens correction. This paper examines differences in stereopsis at 4.5 years of age between the 2 treatment groups. Methods . The Infant Aphakia Treatment Study is a randomized clinical trial involving 12 sites supported by a
Read “Stereopsis Results at 4.5 Years of Age in the Infant Aphakia Treatment Study, American Journal of Ophthalmology” on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
Baseline Characteristics of the Infant Aphakia Treatment
Corneal Changes in Children after Unilateral Cataract
The infant aphakia treatment study: Design and clinical measures at enrollment Journal Article Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
Infant Aphakia Treatment Study definition, categories, type and other relevant information provided by All Acronyms. IATS stands for Infant Aphakia Treatment Study
To the Editor We read with interest the article on 4.5-year visual acuity outcomes for the Infant Aphakia Treatment Study (IATS). 1 This is clearly a well-thought-out and meticulously devised study.
To evaluate sensorimotor outcomes among children in the Infant Aphakia Treatment Study (IATS).Secondary outcome analysis was performed in this randomized, multicenter, clinical trial comparing treatment of unilateral aphakia with a primary intraocular lens (IOL) or …
The Infant Aphakia Treatment Study is a randomized trial that compares the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CLs). The purpose of this study was to compare the outcomes for infants with lens opacity associated with persistent fetal vasculature (PFV) to those without.
Author Manuscript NIH Public Access Measures at Enrollment
Infant Aphakia Treatment Study (IATS) Full Text View
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: • To determine whether infants with a …
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
Surgery for congenital cataracts in early infancy usually includes a primary posterior capsulectomy and an anterior vitrectomy. Initially, most of these infants have aphakia after surgery.
J AAPOS. 2016 Dec;20(6):501-505. doi: 10.1016/j.jaapos.2016.09.014. Epub 2016 Nov 2. Strabismus surgery outcomes in the Infant Aphakia Treatment Study (IATS) at age 5 years.
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
METHODS: The Infant Aphakia Treatment Study is a multicenter clinical trial in which 114 infants with unilateral congenital cataracts were randomized to undergo cataract extraction with contact lens correction or implantation of an intraocular lens. Patching data were collected during the year preceding a visit at age 4.5 years, when both visual acuity and caregiver-reported behavioral
implantation in the infant aphakia treatment study (Ophthalmology. 2017;124:822-827) TO THE EDITOR: In the study by Weakley et al, 1 the authors have evaluated the myopic shift occurring after surgery in children with uniocular congenital cataract enrolled in the Infant Aphakia Treatment Study (IATS).1 The authors have commented on eyes implanted with an intraocular lens (IOL), while they …
Infant Aphakia Treatment Study: Controversy remains regarding the safety and efficacy of lens implantation in infants. Until recently no randomised clinical trials comparing implantation to contact lens correction of aphakia had been carried out. The Infant Aphakia Treat-ment Study (IATS) recently published its 1 year outcomes. 14. Briefly, this prospective, multicentre, randomised clinical
Assessment of adherence to visual correction and occlusion therapy in the Infant Aphakia Treatment Study Carolyn Drews-Botsch a, *, George Cotsonis b, Marianne Celano c, Scott R. Lambert d
The Infant Aphakia Treatment Study (IATS) is a multi-center randomized clinical trial comparing intraocular lens and contact lens correction for monocular aphakia. Infants will be enrolled over a 4 year period. Infants 28 to 210 days of age with a visually significant cataract in one eye are eligible. Cataract surgery will be performed in a standardized fashion by a surgeon who has been
: We analyzed baseline characteristics of the 114 infants enrolled in the Infant Aphakia Treatment Study to determine which were most predictive of visual outcome at 4.5 years of age. All infants underwent cataract surgery between 1 and 7 months of age. Monocular acuity was assessed at 4.5 years of age by a traveling examiner using the Amblyopia Treatment Study HOTV protocol.
Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study By David A. Plager, Michael J. Lynn, Edward G. Buckley, M. Edward Wilson, Scott R. Lambert and Infant Aphakia Treatment Study Group
Motor skills of children with unilateral visual impairment
The Infant Aphakia Treatment Study Contact Lens Experience
Infant Aphakia Treatment Study Group, Lambert SR , Lynn MJ et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4·5 years and clinical findings at age 5 years.
The Infant Aphakia Treatment Study (IATS) is a randomized trial comparing the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CL). The purpose of this study was to compare the outcomes for infants with lens opacity associated
1 April 4, 2017 Lessons learned about cataract surgery in infants from the Infant Aphakia Treatment Study . Summary: The workshop will present the latest findings from the Infant Aphakia Treatment Study
With publication of its 5-year results, the Infant Aphakia Treatment Study (IATS) helped illuminate the way forward for these children. 1 Three of its steering committee investigators review key lessons learned and how they are being translated into clinical practice.
The Infant Aphakia Treatment Study, a randomized clinical trial conducted at 12 different sites, compared IOLs to contact lenses in 114 infants with unilateral congenital cataracts. The infants were between one and six months old when cataract surgery was performed.
for the Infant Aphakia Treatment Study Group5 . By . Abstract . Objective To evaluate parenting stress following infants ’ cataract extraction surgery, and to determine if levels of stress differ between 2 treatments for unilateral congenital cataract in a randomized clinical trial. Methods At surgery, an intraocular lens (IOL) was implanted or children were left aphakic, treated with
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
Methods. The Infant Aphakia Treatment Study is a randomized clinical trial in which the investigators compared intraocular lens (IOL) versus contact lens correction in 114 infants…
Treatment outcomes in malignant glaucoma. Dave P. et al. Use the article you are viewing now to seed an intelligent alerting service that pinpoints the most relevant new articles for you.
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
Study on Children with Cataract Removal Reports Using a
Infant Aphakia Treatment Study (IATS) Study Results
How to Cite. Celano, M., Hartmann, E. E., DuBois, L. G., Drews-Botsch, C. and the Infant Aphakia Treatment Study Group (2015), Motor skills of children with
Infant Aphakia Treatment Study Group, Lambert SR , Lynn MJ et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4·5 years and clinical findings at age 5 years.
Infant Aphakia Treatment Study (IATS) (IATS) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
The Infant Aphakia Treatment Study, a randomized clinical trial conducted at 12 different sites, compared IOLs to contact lenses in 114 infants with unilateral congenital cataracts. The infants were between one and six months old when cataract surgery was performed.
To the Editor We read with interest the article on 4.5-year visual acuity outcomes for the Infant Aphakia Treatment Study (IATS). 1 This is clearly a well-thought-out and meticulously devised study.
TheInfantAphakiaTreatmentStudy(IATS)isamul-ticenter, randomized, controlled clinical trial sponsored by the National Eye Institute. The objective of the study
IOL implantation for infants under 7 months and primary IOL resulted in more adverse events and reoperations Infant Aphakia Treatment Study Group, JAMA Ophthalmol. 2014;132(6):676‐682
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
METHODS: The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract. This report is a secondary outcome analysis of ocular motor data from IATS patients who underwent strabismus surgery prior to age 5 years. RESULTS: Strabismus surgery was …
The Infant Aphakia Treatment Study is a randomized trial that compares the treatment of unilateral congenital cataract with primary intraocular lens (IOL) implantation versus aphakic contact lens (CLs). The purpose of this study was to compare the outcomes for infants with lens opacity associated with persistent fetal vasculature (PFV) to those without.
The Infant Aphakia Treatment Study randomized infants who had surgery for a unilateral cataract between 1 and 7 months of age to either primary intraocular lens or contact lens correction. This paper examines differences in stereopsis at 4.5 years of age between the 2 treatment groups. Methods . The Infant Aphakia Treatment Study is a randomized clinical trial involving 12 sites supported by a
Migration to aphakia and contact lens treatment is the
Contact lens in children with aphakia current scenario
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: • To determine whether infants with a …
Read “Stereopsis Results at 4.5 Years of Age in the Infant Aphakia Treatment Study, American Journal of Ophthalmology” on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study By David A. Plager, Michael J. Lynn, Edward G. Buckley, M. Edward Wilson, Scott R. Lambert and Infant Aphakia Treatment Study Group
The infant aphakia treatment study: Design and clinical measures at enrollment Journal Article Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
The Infant Aphakia Treatment Study Contact Lens Experience, which compared contact lens correction for unilateral aphakia with IOL implantation for children between one and six months of age, showed visual acuity outcomes were similar at age one and four and a half. 4 The rate of adverse events was lower in the study’s contact lens wearers, and few patients in that group required additional
Infant Aphakia Treatment Study, a randomized, multi- center (12) clinical trial comparing the treatment of uni- lateral aphakia in patients under 7 months of age with a
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: •
The Infant Aphakia Treatment Study (IATS) is a multi-center. Therefore. & Wagner. Earlier reports from this study suggest that there is wide variation in the grating VA of children enrolled in this study at 12 months of age. parenting stress instruments designed to capture the particular burdens associated with taking care of an infant with a unilateral cataract are more likely to clarify the
METHODS: The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract. This report is a secondary outcome analysis of ocular motor data from IATS patients who underwent strabismus surgery prior to age 5 years. RESULTS: Strabismus surgery was …
The Infant Aphakia Treatment Study (IATS) is a multi-center randomized clinical trial comparing intraocular lens and contact lens correction for monocular aphakia. Infants will be enrolled over a 4 year period. Infants 28 to 210 days of age with a visually significant cataract in one eye are eligible. Cataract surgery will be performed in a standardized fashion by a surgeon who has been
IOL implantation for infants under 7 months and primary IOL resulted in more adverse events and reoperations Infant Aphakia Treatment Study Group, JAMA Ophthalmol. 2014;132(6):676‐682
4. Parenting Stress in the Infant Aphakia Treatment Study
Infant Aphakia Treatment Study (IATS) Study Results
title = “The infant aphakia treatment study: Design and clinical measures at enrollment”, abstract = “Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
The Infant Aphakia Treatment Study (IATS) is a multi- centre, randomised controlled clinical trial comparing treatment of unilateral cataract in infants aged 1–6 months
METHODS: The Infant Aphakia Treatment Study is a multicenter clinical trial in which 114 infants with unilateral congenital cataracts were randomized to undergo cataract extraction with contact lens correction or implantation of an intraocular lens. Patching data were collected during the year preceding a visit at age 4.5 years, when both visual acuity and caregiver-reported behavioral
There were 114 children enrolled in the study with 57 randomized to each treatment group. In years 2–5 following surgery, the patients were examined by an Infant Aphakia Treatment
With publication of its 5-year results, the Infant Aphakia Treatment Study (IATS) helped illuminate the way forward for these children. 1 Three of its steering committee investigators review key lessons learned and how they are being translated into clinical practice.
Primary intraocular lens (IOL) implantation after congenital cataract surgery in infants has always been controversial. The perception of pediatric ophthalmologists regarding the implantation of IOLs in children has changed radically during the past two decades. With the advances in the surgical instruments and techniques, commercial
Read “Stereopsis Results at 4.5 Years of Age in the Infant Aphakia Treatment Study, American Journal of Ophthalmology” on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
To evaluate sensorimotor outcomes among children in the Infant Aphakia Treatment Study (IATS).Secondary outcome analysis was performed in this randomized, multicenter, clinical trial comparing treatment of unilateral aphakia with a primary intraocular lens (IOL) or …
Assessment of adherence to visual correction and occlusion therapy in the Infant Aphakia Treatment Study Carolyn Drews-Botsch a, *, George Cotsonis b, Marianne Celano c, Scott R. Lambert d
The infant aphakia treatment study: Design and clinical measures at enrollment Journal Article Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
Glaucoma-related adverse events in the Infant Aphakia
Comments on Infant Aphakia Treatment Study 4.5-Year
Recent results of IATS (Infant Aphakia Treatment Study) have reported good compliance with contact lens wear in infants and concluded CL to be ideal substitutes for intraocular lens implantation
Scott R. Lambert, Edward G. Buckley, Carolyn Drews-Botsch, Lindreth DuBois, Eugenie Hartmann, Michael J. Lynn, David A. Plager, M. Edward Wilson, Betsy Bridgman
Infant Aphakia Treatment Study definition, categories, type and other relevant information provided by All Acronyms. IATS stands for Infant Aphakia Treatment Study
The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without intraocular lens (IOL) implantation in 114 children who underwent unilateral cataract surgery at 1 to
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
Assessment of adherence to visual correction and occlusion therapy in the Infant Aphakia Treatment Study Carolyn Drews-Botsch a, *, George Cotsonis b, Marianne Celano c, Scott R. Lambert d
for the Infant Aphakia Treatment Study Group5 CORE
THE INFANT APHAKIA TREATMENT STUDY (IATS)
To evaluate sensorimotor outcomes among children in the Infant Aphakia Treatment Study (IATS).Secondary outcome analysis was performed in this randomized, multicenter, clinical trial comparing treatment of unilateral aphakia with a primary intraocular lens (IOL) or …
Stereopsis Resultsat 4.5 Years of Age in the Infant Aphakia Treatment Study E. EUGENIE HARTMANN, ANN U. STOUT, MICHAEL J. LYNN, KIMBERLY G. YEN, STACEY J. KRUGER, AND
The Infant Aphakia Treatment Study (IATS) is a multicenter, randomized, controlled clinical trial sponsored by the National Eye Institute undertaken to determine whether primary IOL implantation in infants between 1 and 6 months of age with unilateral cataract would result in improved visual outcomes over contact lens correction of aphakia. Half of the 114 infants enrolled in this multicenter
What does IATS mean in Unclassified? This page is about the meanings of the acronym/abbreviation/shorthand IATS in the Miscellaneous field in general and in the Unclassified terminology in particular.
The Infant Aphakia Treatment Study (IATS) is a multi-center, randomized, controlled clinical trial comparing IOL versus contact lens treatment after cataract surgery performed in children with a unilateral congenital cataract between 1 and 6 months of age. This paper describes the design of the study and the clinical findings in these patients at the time of enrollment.
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
Author Manuscript NIH Public Access Measures at Enrollment
Congenital Cataract Advances in Ophthalmology and Optometry
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
PDF Objective: To evaluate parenting stress following infants’ cataract extraction surgery, and to determine if levels of stress differ between 2 treatments for unilateral congenital cataract in
Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. 2 Pages . Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study. Authors. Jagat Ram 2. Jagat Ram. Jaspreet Sukhija. M. Wilson
The Infant Aphakia Treatment Study, a randomized clinical trial conducted at 12 different sites, compared IOLs to contact lenses in 114 infants with unilateral congenital cataracts. The infants were between one and six months old when cataract surgery was performed.
METHODS: The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract. This report is a secondary outcome analysis of ocular motor data from IATS patients who underwent strabismus surgery prior to age 5 years. RESULTS: Strabismus surgery was …
Infant Aphakia Treatment Study (IATS) (IATS) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
The Infant Aphakia Treatment Study (IATS) is a multi-center randomized clinical trial comparing intraocular lens and contact lens correction for monocular aphakia. Infants will be enrolled over a 4 year period. Infants 28 to 210 days of age with a visually significant cataract in one eye are eligible. Cataract surgery will be performed in a standardized fashion by a surgeon who has been
implantation in the infant aphakia treatment study (Ophthalmology. 2017;124:822-827) TO THE EDITOR: In the study by Weakley et al, 1 the authors have evaluated the myopic shift occurring after surgery in children with uniocular congenital cataract enrolled in the Infant Aphakia Treatment Study (IATS).1 The authors have commented on eyes implanted with an intraocular lens (IOL), while they …
Read “Stereopsis Results at 4.5 Years of Age in the Infant Aphakia Treatment Study, American Journal of Ophthalmology” on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
Lessons Learned from the Infant Aphakia Treatment Study A multicenter randomized clinical trial funded by the National Eye Institute Scott R. Lambert, M.D.
The Infant Aphakia Treatment Study (IATS) is a multi- centre, randomised controlled clinical trial comparing treatment of unilateral cataract in infants aged 1–6 months
Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study By David A. Plager, Michael J. Lynn, Edward G. Buckley, M. Edward Wilson, Scott R. Lambert and Infant Aphakia Treatment Study Group
The infant aphakia treatment study: Design and clinical measures at enrollment Journal Article Objective: To compare the use of contact lenses and intraocular lenses (IOLs) for the optical correction of unilateral aphakia during infancy.
(PDF) Parenting Stress in the Infant Aphakia Treatment Study
Congenital Cataract Advances in Ophthalmology and Optometry
Download PDF. Although contact lenses have been used for decades to correct vision in children after cataract surgery, prospective data on adherence to lens wear are limited. In a secondary analysis of the Infant Aphakia Treatment Study, Cromelin et al.
Purpose To report strabismus surgery frequency and outcomes after monocular infantile cataract surgery with or without IOL implantation. Methods The Infant Aphakia Treatment Study (IATS) is a randomized, multicenter clinical trial comparing treatment of aphakia with a primary IOL or contact lens in 114 infants with a unilateral congenital cataract.
Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR, Infant Aphakia Treatment Study G Complications in the First 5 Years Following Cataract Surgery in Infants With and Without Intraocular Lens Implantation in the Infant Aphakia Treatment Study.
The Infant Aphakia Treatment Study Contact Lens Experience, which compared contact lens correction for unilateral aphakia with IOL implantation for children between one and six months of age, showed visual acuity outcomes were similar at age one and four and a half. 4 The rate of adverse events was lower in the study’s contact lens wearers, and few patients in that group required additional
To the Editor We read with interest the article on 4.5-year visual acuity outcomes for the Infant Aphakia Treatment Study (IATS). 1 This is clearly a well-thought-out and meticulously devised study.
ataract Editorial TOUCH MEDICAL MEDIA 21 The Infant Aphakia Treatment Study (IATS) was a randomized, multicenter clinical trial that compared visual outcomes at age 4.5 years with or without
Recent results of IATS (Infant Aphakia Treatment Study) have reported good compliance with contact lens wear in infants and concluded CL to be ideal substitutes for intraocular lens implantation
Surgery for congenital cataracts in early infancy usually includes a primary posterior capsulectomy and an anterior vitrectomy. Initially, most of these infants have aphakia after surgery.
Stereopsis Resultsat 4.5 Years of Age in the Infant Aphakia Treatment Study E. EUGENIE HARTMANN, ANN U. STOUT, MICHAEL J. LYNN, KIMBERLY G. YEN, STACEY J. KRUGER, AND
How to Cite. Celano, M., Hartmann, E. E., DuBois, L. G., Drews-Botsch, C. and the Infant Aphakia Treatment Study Group (2015), Motor skills of children with
Infant aphakia treatment study17 Lambert et al. (Infant Aphakia Treatment Study Group) performed a randomized, multicenter (12 sites) clinical trial in infants with unilateral congenital cataract, assigned to undergo cataract surgery between 1 and 6 months of age, either with or without primary IOL implantation. CLs were used to correct aphakia in patients who did not receive IOLs. No
The Infant Aphakia Treatment Study (IATS) is a randomized, controlled multi-center clinical trial with the following objectives: •
EYENET MAGAZINE • 33 M. Edward Wilson Jr., MD Infant Aphakia: Putting Study Results Into Practice A t one time, “The possibility of restoring the vision in an eye
Ophthalmology. 2017 May;124(5):730-733. doi: 10.1016/j.ophtha.2017.01.010. Epub 2017 Feb 10. Globe Axial Length Growth at Age 5 Years in the Infant Aphakia Treatment Study.
Infant Aphakia Treatment Study definition, categories, type and other relevant information provided by All Acronyms. IATS stands for Infant Aphakia Treatment Study